IFOB test (immunological stool test)
IFOB test (immunological stool test)
Simple and non-invasive colorectal cancer screening
The iFOBT (immunological fecal occult blood test ) is a modern, scientifically based test for the early detection of colorectal cancer . It detects occult blood in the stool , which can indicate changes in the intestine , such as colorectal cancer. The test is discreet and convenient – you can easily perform it at home .
The test uses antibodies to detect human hemoglobin and is therefore significantly more specific than older methods. It is a key component of colorectal cancer screening , especially for those aged 50 and over , but is also increasingly used in younger people.
Advantages of the iFOBT:
- Non-invasive: No procedure required, simply applicable at home.
- Fast results: Within a few business days
- Clinical support: We accompany you every step of the way.
If blood is found in the iFOBT stool test , this is a warning sign – a colonoscopy for clarification is then strongly recommended.
Konnte die Abholverfügbarkeit nicht laden

Every second colorectal cancer diagnosis is made too late¹
Early detection saves lives : In Germany, a new case of colorectal cancer is diagnosed every 8 minutes – often only at an advanced stage. Regular stool tests offer an effective way to detect early warning signs.
From the age of 50 , statutory health insurance companies generally cover the costs of colorectal cancer screening , which includes the immunochemical fecal occult blood test (iFOBT) . Reimbursement requires obtaining the test through a doctor's office.
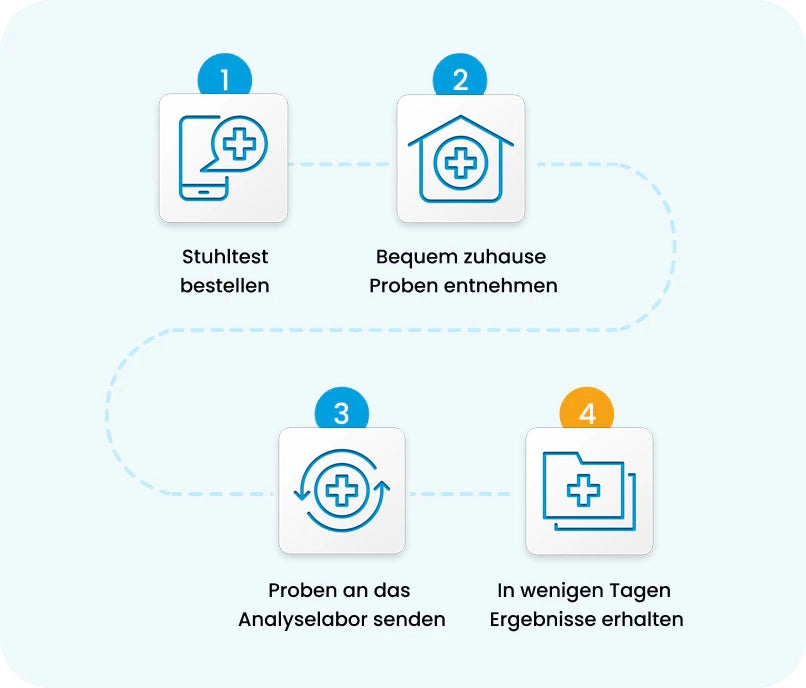
Colorectal cancer screening is that simple at the European Oncology Lab
-

Stool test online
order -

Comfortable at home
Take samples -
Samples to our laboratory
return -

Results received within a few days
How does the blood in stool test work?
The iFOBT is a test for the early detection of colorectal cancer that reliably detects occult (i.e., invisible) blood in stool . Unlike classic tests such as the guaiac test, the immunochemical fecal occult blood test (iFOBT) only reacts to human blood, which significantly increases its accuracy.
The procedure is straightforward: Take a stool sample at home , seal it in the sample tube and send it to our certified laboratory.
An abnormal result can indicate precancerous lesions or other changes in the intestine . This stool test is a low-threshold and effective alternative, especially for people who do not want to undergo a colonoscopy .

iFOBT compared to other stool tests
The iFOBT is a well-established and widely used method. However, newer methods are now available, such as tumor DNA stool tests . These analyze genetic material in stool and can, in some cases, detect colorectal cancer in its early stages – even without the presence of blood.
The advantages of the iFOBT are its ease of use, good availability, and low cost. This makes the stool test ideal for regular preventative care.
Tumor DNA tests such as ColoAlert, on the other hand, offer a higher level of sensitivity, but are more expensive and are not always covered by health insurance.
Conclusion : The iFOBT stool test remains an important tool in colorectal cancer screening – especially when used regularly.

Our recommendation: ColoAlert for earlier colorectal cancer detection
The IFOB test is a good start and certainly better than doing nothing. However, analyzing for hidden blood in stool does not detect all tumors in time . ColoAlert is a new stool test that examines stool samples for the presence of tumor DNA – thus identifying affected individuals at earlier stages.⁴⸴⁵ ColoAlert is reimbursable for those with private health insurance.
Let customers speak for us
from 130 reviewsDieser Test erspart mir schon seit Jahren eine Darmspiegelung!
Und letztes Jahr wurde - ebenfalls durch den Test - bei meinem Mann Darmkrebs im Frühstadium entdeckt. Nach einer OP und Chemotherapie in Tablettenform, welche er zu Hause durchführen konnte, gilt er heute schon wieder krebsfrei.
Ich empfehle diesen Test immer wieder Freunden und Bekannten!
Wer eine Alternative zur Coloskopie als Darmkrebs-Prophylaxe sucht, rate ich zum ColoAlert-Test. Neben dem iFOBT erfährt man, ob im Darm OncoGene nachgewiesen werden können, die bei einer Carcinom-Entwickung ein aggressives Wachstum voraus sagen. Der Test ist so einfach wie der iFOBT alleine und wird nur zu Hause vorgenommen. - Die Kommunikation mit dem European Oncology Lab - Team kann ich nur als hervorragend bewerten!
Es ging super schnell, innerhalb weniger Tage habe ich mein Ergebnis erhalten. Die Erklärung des Befundes war detailliert und verständlich. Ich habe ein besseres Gefühl. Vielen lieben Dank. Ich habe diesen Test weiter empfohlen und ihn auch für meine Mutter bestellt.
Mir gefällt vor allem die schnelle und leicht verständliche Auswertung. Fühle mich jetzt viel sicherer.
Das Testmaterial war super, aber der Rückversand war etwas umständlich, weil ich aus Spanien versenden musste. Insgesamt aber sehr zufrieden.
Das ist bereits mein zweiter Test und wieder war alles reibungslos. Klare Empfehlung meinerseits!
Rasche und sehr gute verständliche Erklärung der Untersuchungsergebnisse! Einfach gesamtbildlich betrachtet hervorragend und unbedingt weiterzuempfehlen! Die Weiterleitung an meine Krankenkasse ist am Laufen!

Why the immunochemical fecal occult blood test (iFOBT) is also useful before the age of 50.
Colorectal cancer is no longer a disease that only affects older people. Studies show that the number of diagnoses in younger people between the ages of 20 and 49 is steadily increasing.² Unfortunately, awareness of the disease is often lacking in this age group, which can lead to symptoms being overlooked or misinterpreted .
The IFOB test offers you the opportunity to detect signs of colorectal cancer – simply and precisely. Especially if additional risk factors such as a family history of the disease, an unhealthy diet, or lack of exercise are present,³ screening before the expected age can be beneficial.
Frequently Asked Questions
Who should make provisions?
Everyone aged 50 and over – but also younger people with symptoms such as blood in the stool or a family history of the disease – should think about colorectal cancer screening .
Is there anything I need to do before the blood in stool test?
No – no diet or special preparation is required for the iFOBT .
What happens if the test is positive?
A positive result indicates occult blood in the stool . This does not automatically mean colon cancer , but a colonoscopy for clarification is important.
How often should the iFOBT be performed?
Current guidelines for colorectal cancer screening recommend an IFOBT test every two years from the age of 50 – especially if no colonoscopy is performed.
Is the iFOBT covered by health insurance?
Yes, from the age of 50, the test is covered by statutory health insurance every two years as part of colorectal cancer screening . However, the test must be obtained through a doctor's office.
What to do if you experience anxiety after a positive IFOBT test?
An abnormal result is no reason to panic. There are various causes for blood in the stool . It's important to take the next step – the colonoscopy – seriously to get a clear answer.
References
1) Robert Koch Institute and the Association of Population-Based Cancer Registries in Germany (2023). Cancer in Germany for 2019/2020, 14th edition. 2) Vuik, FE, Nieuwenburg, SA, Bardou, M., Lansdorp-Vogelaar, I., Dinis-Ribeiro, M., Bento, MJ, Zadnik, V., Pellisé, M., Esteban, L., Kaminski, MF, Suchanek, S., Ngo, O., Májek, O., Leja, M., Kuipers, EJ, & Spaander, MC (2019). Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut, 68(10), 1820–1826. https://doi.org/10.1136/gutjnl-2018-317592 3) Johnson, CM, Wei, C., Ensor, JE, Smolenski, DJ, Amos, CI, Levin, B., & Berry, DA (2013). Meta-analyses of colorectal cancer risk factors. Cancer Causes & Control: CCC, 24(6), 1207–1222. doi.org/10.1007/s10552-013-0201-5 . 4) Krammes, L., Mahmood, HA, Frondorf, FMB, Scholz, CF, Becker, P., Maharjan, S., Sever, AE, Garapati, SV, Balasubramaniam, A., Knabe, MJ, Eidens, MR, Dollinger, MM (2025). State-of-the-Art Colorectal Cancer and Advanced Precancerous Lesion Screening: a Multitarget Stool DNA Test, Clinical Laboratory, 71(1), 34-39. doi.org/10.7754/Clin.Lab.2024.240620 ; Dollinger, MM, Behl, S., & Fleig, WE (2018). Early Detection of Colorectal Cancer: a Multi-Center Pre-Clinical Case Cohort Study for Validation of a Combined DNA Stool Test. Clinical Laboratory, 64(10), 1719-1730. doi.org/10.7754/Clin.Lab.2018.18052 5) Gies, A., Cuk, K., Schrotz-King, P., & Brenner, H. (2018). Direct Comparison of Diagnostic Performance of 9 Quantitative Fecal Immunochemical Tests for Colorectal Cancer Screening. Gastroenterology, 154(1), 93-104. doi.org/10.1053/j.gastro.2017.09.018